Rhode Island companies engage in helmet ventilation research with innovators in Bangladesh
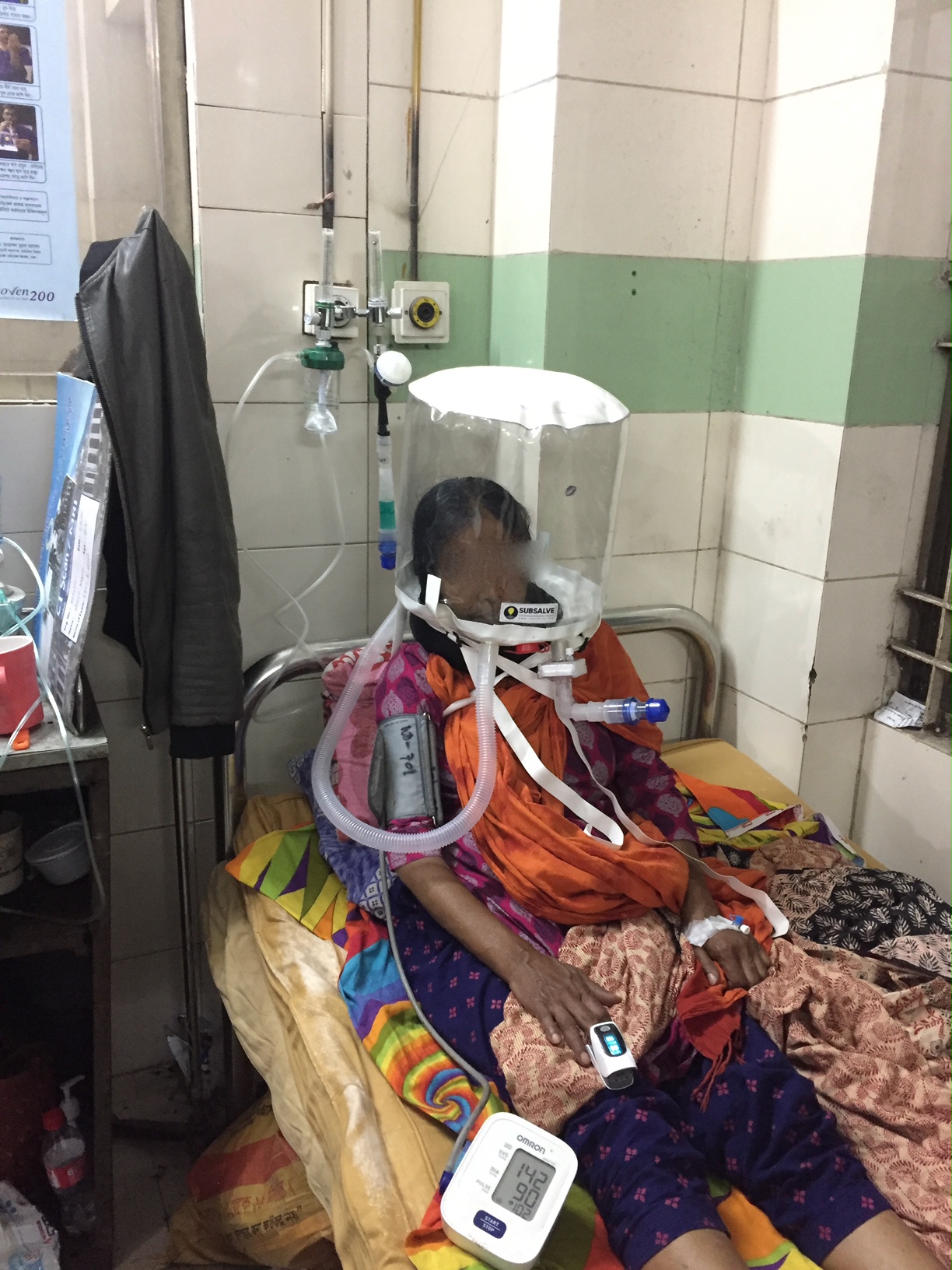
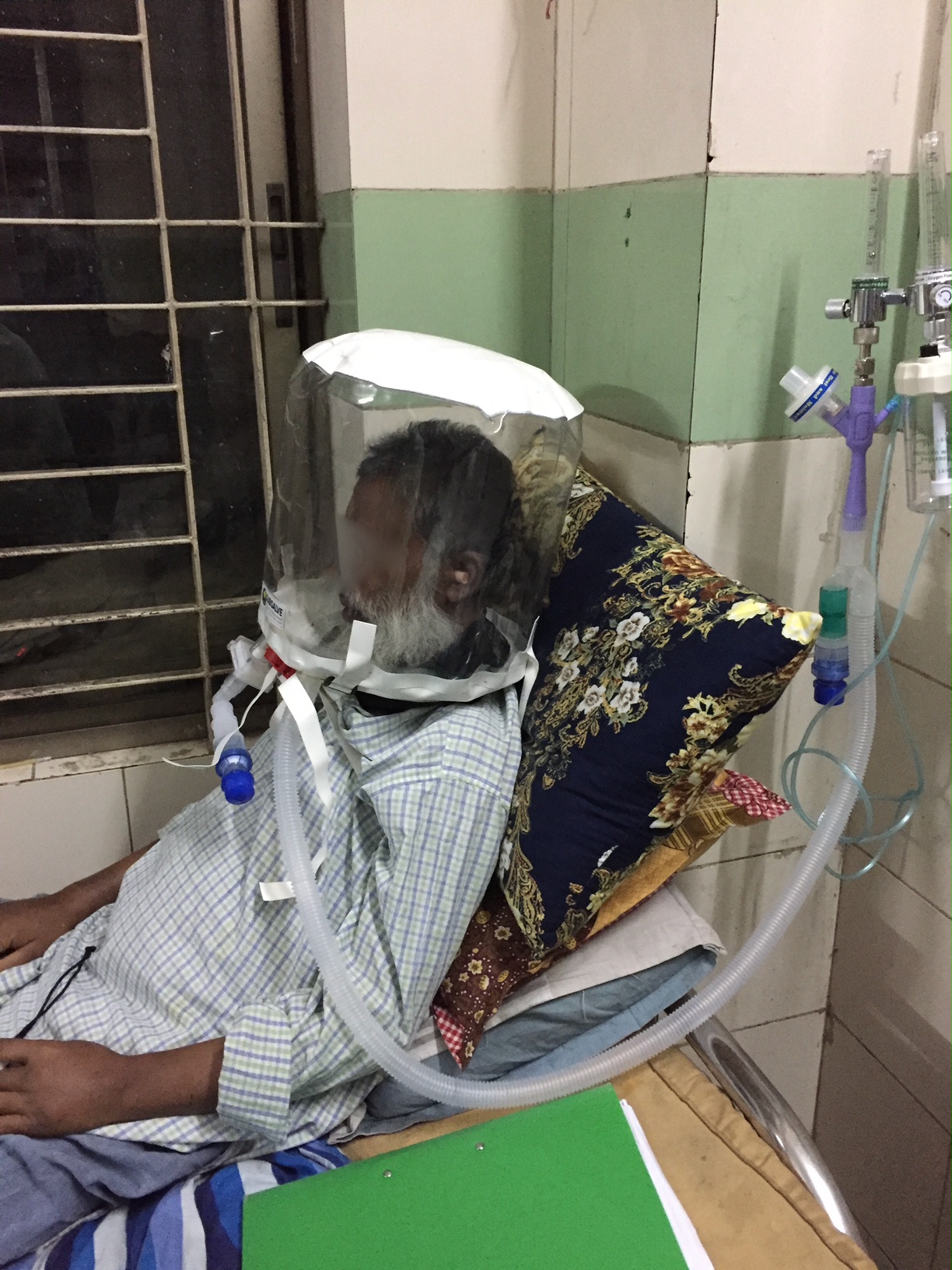
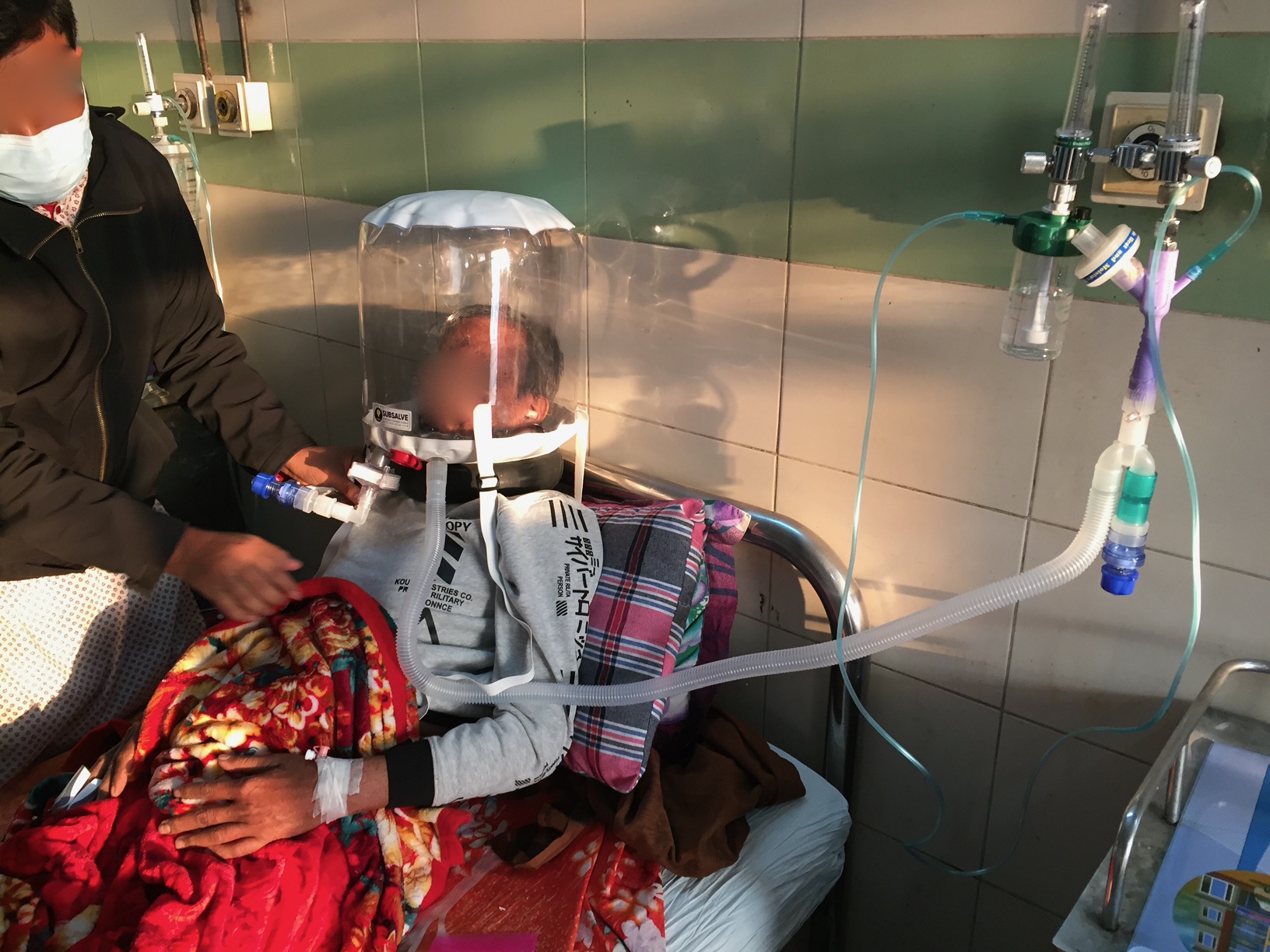
Barrington, Rhode Island Feb 8, 2021 (Issuewire.com) - A new low-cost medical device called OXYJET, developed by a team of researchers at Bangladesh University of Engineering and Technology (BUET) is showing promising results in a clinical trial aimed at treating hypoxemic patients from Covid-19. The team, led by Dr. Taufiq Hasan, an Assistant Professor of Biomedical Engineering at BUET, reports that using OXYJET non-invasive ventilation with the Rhode Island manufactured SUBSALVE OXYGEN TREATMENT HOOD results in significantly improving the oxygen saturation by >11% within 15 minutes when trialed on a small number of patients.
The OXYJET is a precision venturi device optimized for the delivery of Hood/helmet Positive Airway Pressure (HPAP). This setup represents one mode of delivering helmet non-invasive ventilation (NIV) that has been used in Italy and elsewhere for decades to treat Acute Respiratory Distress Syndrome, or ARDS, and with much success (Ref 1). OXYJET is capable of providing 65 liters/min of oxygenated airflow with up to 95% oxygen concentration.
Recently, Helmet NIV has been used specifically for Covid-19 patients as a proactive step to reduce required intubations and more sophisticated ventilators (Ref 2). Intubating a patient requires an endotracheal tube and a degree of sedation - both are invasive and the patient outcomes are not favorable. Recent reports suggest that mortality once a Covid-19 patient is on a ventilator is very high, perhaps as high as 80% (Ref 3). According to University of Chicago studies, intubations can be reduced by as much as 40% when helmet based ventilation techniques are utilized proactively (Ref 4). Until the pandemic, all helmet ventilation for respiratory distress administered in the US has been done 'off-label' as the FDA cleared indication for this technology has previously been for hyperbaric medicine. This has presented numerous regulatory challenges and therefore limited production scalability that the US needed to overcome to facilitate more widespread adoption.
As part of their Covid-19 response, Subsalve USA of Quonset Rhode Island, a 40-year manufacturer of performance engineered inflatable products for undersea, defense, and other markets rapidly designed and established high volume production of the SUBSALVE OXYGEN TREATMENT HOOD in response to the short supply of existing helmet ventilation devices on the market. At present, Subsalve is recognized as having the highest production capacity for these devices globally. Since project inception in April 2020, the company has delivered more than 20,000 devices globally, with many directed towards humanitarian aid in resource limited countries where sophisticated ventilators are non-existent.
The US FDA announced Emergency Use Authorization (EUA) for the Subsalve Oxygen Treatment Hood to be used for non-invasive ventilation of Covid-19 patients on August 4th, 2020.
Subsalve teamed up with Lombardi Undersea LLC, a diving technology research company, to market the new device. The SUBSALVE OXYGEN TREATMENT HOOD technology is ideally suited for Covid-19 as it allows clinicians to control airway pressure, increase therapeutic oxygen levels, and mitigate virus exposure for healthcare workers.
Michael Lombardi stated, "we got in this to fight the good fight - I recognized very early that diving technology could make a difference for Covid-19 and felt a social responsibility to do what I could. Covid-19 has presented the perfect storm for our team's capabilities - we are aggressively supporting emerging research (and development) with multiple partners - this remains a serious pandemic and requires decisive solutions."
Among the challenges with helmet ventilation are understanding the requirements to run them. Lombardi states, "Helmet/hooded ventilation is incredibly simple, but mistakenly perceived as complex since the community has in large part been gravitating to sophisticated ventilation modalities. The hoods can be operated with very simple and inexpensive high flow air and oxygen sources already in place in most hospitals and even rural clinics around the world."
Dr. Hasan said, "The OXYJET CPAP has a precision venturi valve that uses a pressurized oxygen source to entrain room air to create a high-flow, a mechanism known as jet-mixing. The device works without electric power making it ideal for low-resource hospital settings as in Dhaka Medical College Hospital (DMCH), where the clinical trial is being conducted."
DMCH, a leading public hospital in Bangladesh, has a capacity of about 600 patients in the Covid-19 and suspected Covid-19 wards. The hospital has a centralized oxygen source and patient outlets that can provide up to 15 liters/min of oxygen can be provided using a traditional nasal cannula or a mask. But on any given day, there are patients who are in need of a higher level of care in the ICU, where there is a limited number of high-flow nasal oxygenation (HFNO), positive pressure ventilator (CPAP or BiPAP), mechanical ventilators are available. With only 24 ICU beds in DMCH, these patients and their relatives helplessly wait for an ICU bed to become available - or pray that the 15 liters/min of oxygen will be sufficient to maintain the oxygen level of the patient.
Dr. Hasan stated - "The OXYJET CPAP was built exactly to address this gap by introducing a simple positive pressure ventilation system to patients admitted to the Covid Wards - and prevent them from needing an ICU in the first place."
The OXYJET core team members, Kawsar Ahmed, Meemnur Rashid, Kaisar Ahmed, and Farhan Muhib, are all BME undergraduate students. The OXYJET CPAP clinical trials are underway with oversight of the National Research and Ethics Committee (NREC) of the Bangladesh Medical Research Council (BMRC). Given that the OXYJET CPAP costs only about $50 while HFNO devices can cost up to $5000, the team believes that even establishing non-inferiority (or equivalence) of OXYJET compared HFNO will be a significant milestone in terms of overall impact (Ref 5).
More Information
Project Landing Page: www.oxygentreatmenthoods.com
BUET OXYJET: http://mhealth.buet.ac.bd/oxyjet/
Subsalve USA: www.subsalve.com
References
- Reference 1:
https://www.dailymail.co.uk/news/article-8134247/Bubble-helmets-used-Italian-doctors-boost-survivalrates.html
- Reference 2:
https://www.uchicagomedicine.org/forefront/patient-care-articles/helmet-based-ventilation-is-superior-to-face-mask-for-patients-with-respiratory-distress
- Reference 3: https://www.physiciansweekly.com/mortality-rate-of-covid-19-patients-on-ventilators/
- Reference 4: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6005726/
- Reference 5: https://clinicaltrials.gov/ct2/show/NCT04681859
Media Contact
Ocean Opportunity Inc.
Source :Lombardi Undersea LLC
This article was originally published by IssueWire. Read the original article here.