- Reproductive Fertility Center in Corona, CA to offer novel investigational platform for fertility treatment
Gameto, a clinical-stage biotech company developing iPSC-based therapies to transform fertility care, today announced that the Reproductive Fertility Center in Corona, CA, led by Dr. Daniel Williams, is now open for patient enrollment in Gameto’s first clinical study of Fertilo in the United States.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250424888148/en/
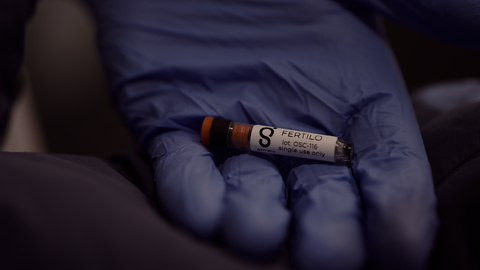
Photo caption: Gameto's Fertilo investigational product made of ovarian support cells (OSCs) in a vial. Photo credit: Gameto
Fertilo is an investigational platform designed to reduce the burden of ovarian stimulation for women undergoing fertility treatment. By using engineered ovarian support cells to promote egg maturation outside of the body, Fertilo aims to shorten or eliminate the need for weeks of hormone injections typically required in conventional in vitro fertilization (IVF) cycles.
The trial at Reproductive Fertility Center represents a significant milestone as Gameto expands Fertilo’s clinical development across leading fertility centers in the U.S. In addition to this center in California, Gameto expects to open over a dozen additional clinical sites in the coming weeks as part of its U.S. Fertilo study rollout. New sites are anticipated across key states including Florida, Texas, New York, and Connecticut, with more locations to follow.
“Opening our first U.S. clinical site for our pivotal study, is an exciting step forward for Gameto and for the future of fertility care,” said Dr. Dina Radenkovic, CEO & Co-Founder, Gameto. “We are proud to partner with Dr. Williams and his outstanding team at Reproductive Fertility Center to offer women a new investigational option aimed at making fertility treatment faster, easier, and less invasive.”
The Fertilo study will evaluate the ability of engineered ovarian support cells to mature patient eggs ex vivo, with the goal of achieving fertilization and embryo development after a much shorter period of ovarian stimulation. Patients participating in the study will receive personalized monitoring and care at Reproductive Fertility Center, one of Southern California’s leading fertility clinics.
“We are excited to be the first clinical site in the U.S. enrolling patients in this innovative study,” said Dr. Daniel Williams, Medical Director of Reproductive Fertility Center. “Our mission has always been to provide patients with access to the latest advances in reproductive medicine. Fertilo has the potential to offer a safer, simpler alternative for women who want to preserve their fertility or start a family. And with the expertise of our clinical team, including Dr. James P. Lin and Dr. Susan Nasab, we are proud to contribute to advancing this important research.”
Gameto recently published a preprint on medRxiv demonstrating that Fertilo can effectively promote egg maturation, leading to a higher number of viable embryos and significantly improved pregnancy success rates. In mini-stimulation (mini-stim) cycles supplemented with Fertilo, patients achieved a 44% pregnancy rate per cycle after the first embryo transfer, more than double the success rate typically observed with conventional in vitro maturation (IVM), which stands at 20%. Patients treated with Fertilo also had more viable embryos available for transfer, further enhancing their chances of conception.
The company plans to open additional clinical sites in its pivotal U.S. Phase 3 clinical trial (NCT06858111) of Fertilo, a first-in-class iPSC-derived therapy. Individuals interested in joining the study can learn more at clinicaltrials.gov.
About Fertilo
Fertilo is Gameto’s ovarian support cell (OSC) product that matures eggs outside the body using iPSC-derived cells. By mimicking the natural ovarian environment in vitro, Fertilo enables replacement of ~80% of hormone injections and shortens IVF or egg freezing cycles from 10-14 days to just 2-3 days. This approach offers a potentially safer, less invasive alternative to traditional IVF, significantly reducing patient burden and the risk of ovarian hyperstimulation.
About Gameto
Gameto is a biotechnology company developing novel solutions for women's health, starting with infertility. Gameto brings together an experienced scientific management team with the vision and passion to develop a product suite to support women throughout their reproductive journeys. Gameto's lead program, Fertilo, aims to make IVF and egg freezing shorter, safer, and more accessible through reduced hormonal injections by maturing eggs outside of the body. Gameto is led by physician-turned-entrepreneur Dina Radenkovic as CEO and serial entrepreneur and founder of one of North America's largest fertility networks Prelude Fertility, Martin Varsavsky, as Chairman. For more information, go to gametogen.com or follow us on Twitter and Instagram @gametogen and on LinkedIn.
View source version on businesswire.com: https://www.businesswire.com/news/home/20250424888148/en/
Contacts
Investor
Kylie Jordan
kylie@gametogen.com
Media
Kimberly Ha
KKH Advisors
kimberly.ha@kkhadvisors.com
917-291-5744
